No
serious AEs were
observed2
In the studies, there was a low discontinuation rate (1.3%) due to adverse events (AEs)2
1.3% Discontinued VUITY® due to AEs2
(5/375) vs 0.5% vehicle (2/374)
0.5% Discontinued VUITY® due to headache2
(2/375) vs 0.3% vehicle (1/374)
Most AEs were reported as
mild and transient
in clinical trials.2
Most AEs were reported as mild and transient in clinical trials.2
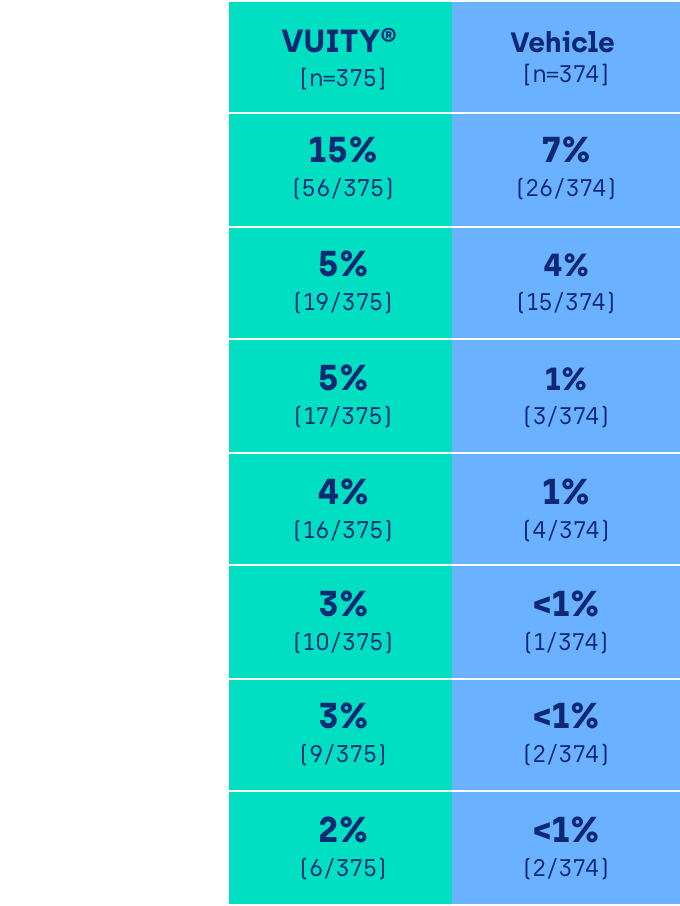
The majority of treatment-emergent adverse events (TEAEs) were deemed by the investigator to be mild in severity. TEAEs of moderate severity were reported for 4.0% of participants [15/375] in the VUITY® group and 2.4% of participants [9/374] in the vehicle group. Severe TEAEs were reported for 1.1% [4/375] of participants in the VUITY® group and 0.5% [2/374] of participants in the vehicle group.2
Rate of TEAEs in GEMINI 1 and GEMINI 2 (pooled data)1,2
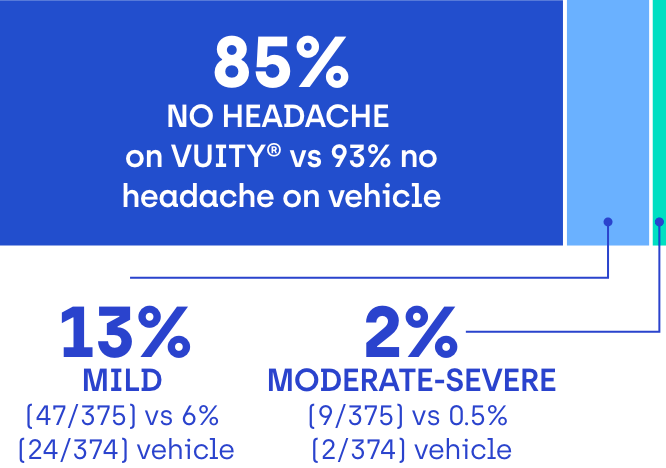
85% of VUITY® participants in clinical trials didn’t experience headaches2
Most headaches were reported as mild and transient. Participants were asked if they experienced a headache and, if so, to rate its severity at every study visit.2
Warnings and Precautions
Rare cases of retinal detachment and retinal tear have been reported with miotics, including VUITY®. Individuals with pre-existing retinal disease are at increased risk. Therefore, examination of the retina is advised in all patients prior to the initiation of therapy. Patients should be advised to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss.1
Post-marketing data
The following adverse reactions have been identified during postapproval use of VUITY®. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to VUITY® exposure. Eye disorders: vitreous detachment, vitreomacular traction, retinal tear, retinal detachment.1
