Not an actual patient
Improved
near and intermediate vision
without compromising distance vision.1
without compromising
distance vision.1
Primary endpoint was the proportion of participants gaining ≥3 lines in mesopic, high-contrast, binocular DCNVA without losing more than 1 line (5 letters) of corrected distance visual acuity (CDVA) with the same refractive correction at Day 30, Hour 3.1 Change from baseline in photopic, high-contrast, binocular distance-corrected intermediate visual acuity (DCIVA) at Day 30, Hour 3 was a prespecified secondary endpoint.3
Primary endpoint was the proportion of participants gaining ≥3 lines in mesopic, high-contrast, binocular DCNVA without losing more than 1 line (5 letters) of corrected distance visual acuity (CDVA) with the same refractive correction at Day 30, Hour 3.1 Change from baseline in photopic, high-contrast, binocular distance-corrected intermediate visual acuity (DCIVA) at Day 30, Hour 3 was a prespecified secondary endpoint.3
The data in the chart below shows the results for GEMINI 1. Click the GEMINI 2 button below to see the results for that study.
Significantly more participants
gained ≥3 lines
in mesopic (low light) DCNVA
without losing more than 1 line (5 letters) of CDVA at Day 30, Hour 3 vs vehicle.*1
*GEMINI 1: 31% of participants on VUITY® vs 8% vehicle; p<0.01; intent-to-treat (ITT) population.1 GEMINI 2: 26% of participants on VUITY® vs 11% vehicle; p<0.01; ITT population.1
Proportion of participants achieving ≥ 3-line gain in mesopic DCNVA at Day 30 (ITT population)1
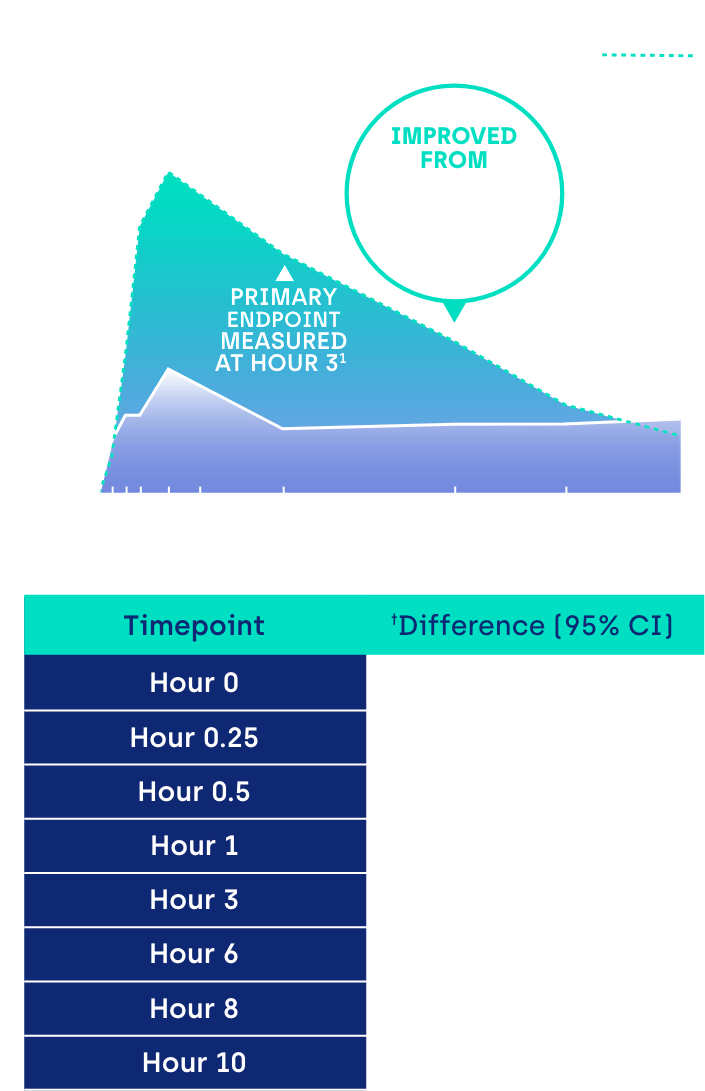
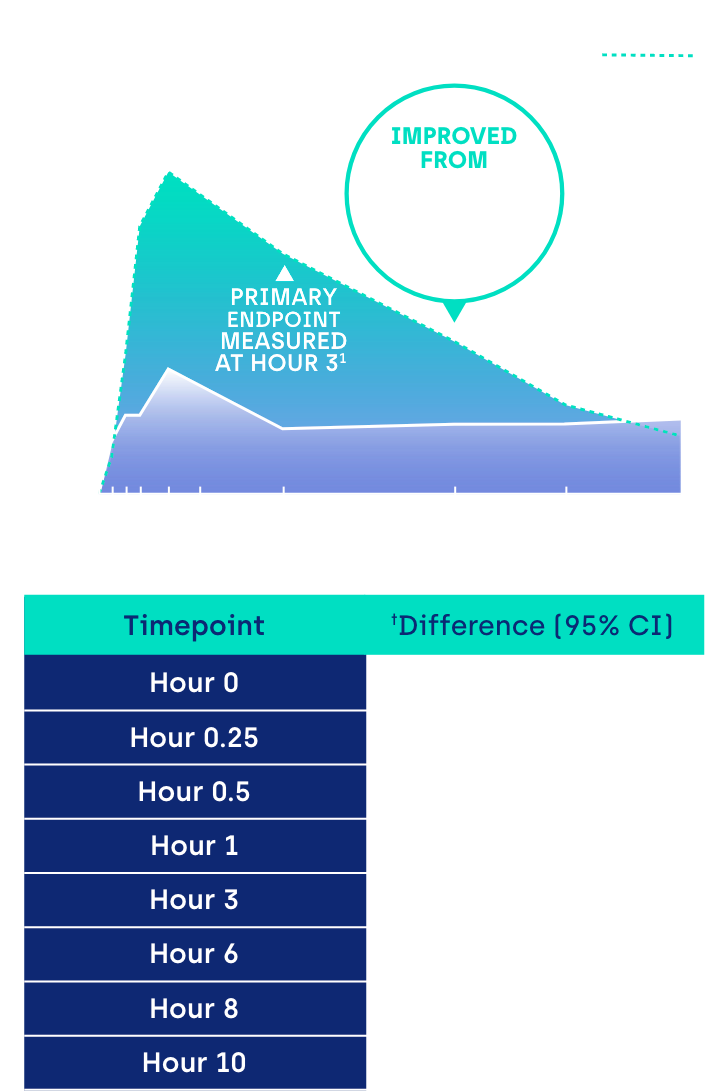
*GEMINI 1: 31% of participants on VUITY® vs 8% vehicle; p<0.01; intent-to-treat (ITT) population.1 GEMINI 2: 26% of participants on VUITY® vs 11% vehicle; p<0.01; ITT population.1
Proportion of participants achieving ≥ 3-line gain in mesopic DCNVA at Day 30 (ITT population)1
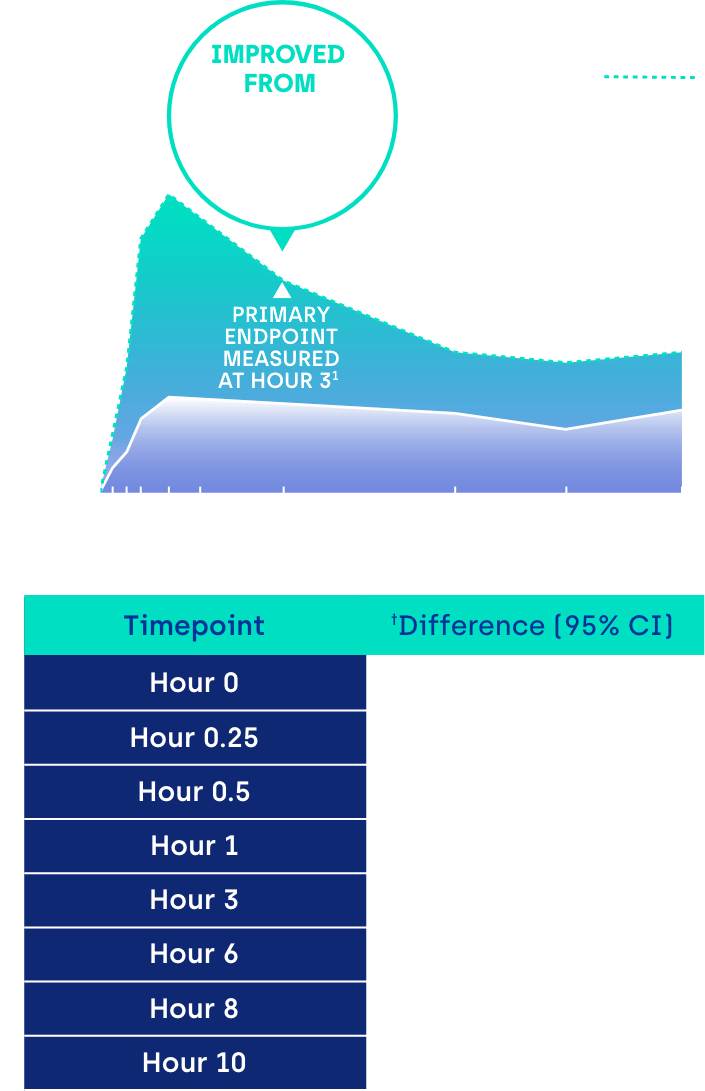
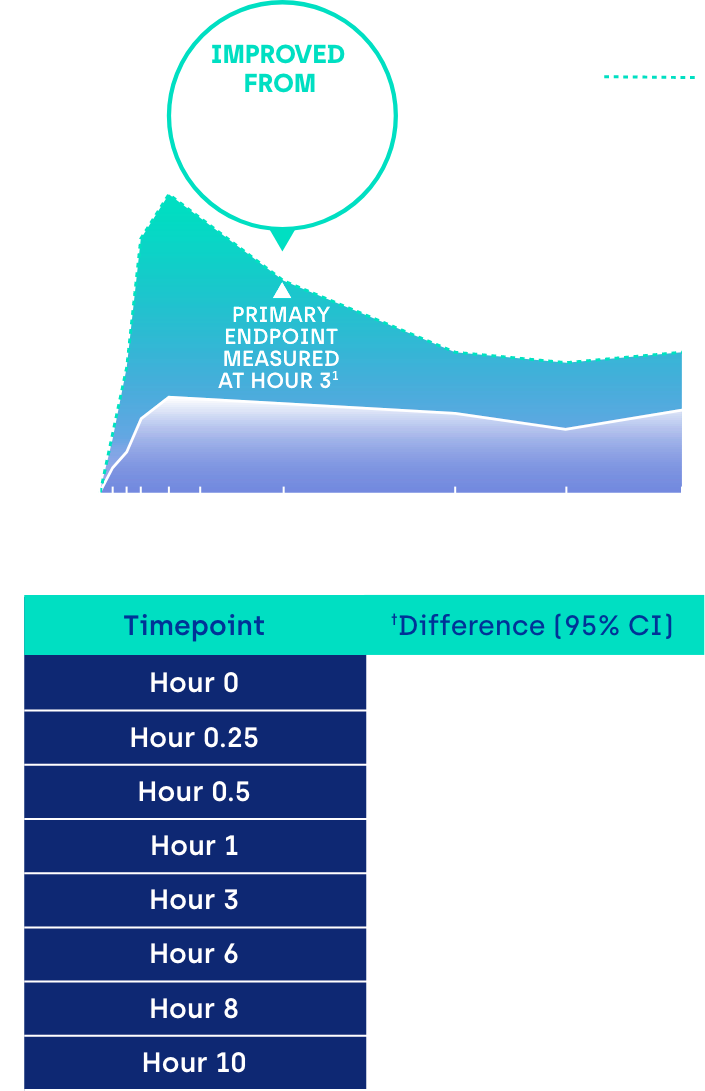
CI: confidence interval.
Evaluation of participants achieving
20/40 or better in DCNVA
photopic high-contrast, binocular conditions at Day 30, Hour 34
~9/10
participaints on
VUITYTM achieved
≥ 20/40
Prespecified secondary endpoint at Day 30, hour 3 (prespecified secondary endpoint)3
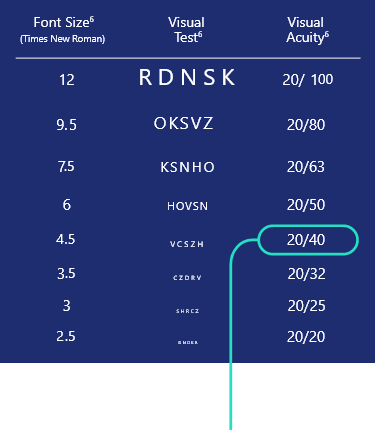
Fonts not actual size.
In post-hoc analysis:4
1/3 VUITYTM participants achieved 20/20 DCNVA without losing more than 5 letters of CDVA on Day 30, Hour 1^
GEMINI 1: VUITYTM (n=161), vehicle (n=153). GEMINI 2: VUITYTM (n=193), vehicle (n=119)
At baseline in GEMINI 1, 40.5% (vs 38.8% vehicle) of participants in the VUITYTM group had 20/40 to 20/60 mesopic, high contrast binocular DCNVA.
At baseline in GEMINI 1, 40.5% (vs 38.8% vehicle) of participants in the VUITY™ group had 20/40 to 20/60 mesopic, high-contrast binocular DCNVA. At baseline in GEMINI 2, 48.6% (vs 48.8% vehicle) of participants in the VUITY™ group had 20/40 to 20/60 mesopic, high-contrast binocular DCNVA.
~9/10
participants on
VUITY® achieved
≥ 20/40
DCNVA at Day 30, Hour 3 (prespecified secondary endpoint)4
- 84.5% in GEMINI 1 vs 71.9% vehicle4
- 90.2% in GEMINI 2 vs 77.8% vehicle4

Fonts not actual size.
Fonts not actual size.
GEMINI 1: VUITY® (n=161), vehicle (n=153). GEMINI 2: VUITY® (n=193), vehicle (n=198).
At baseline in GEMINI 1, 40.5% (vs 38.8% vehicle) of participants in the VUITY® group had 20/40 to 20/60 mesopic, high contrast binocular DCNVA.
At baseline in GEMINI 1, 40.5% (vs 38.8% vehicle) of participants in the VUITY® group had 20/40 to 20/60 mesopic, high-contrast binocular DCNVA. At baseline in GEMINI 2, 48.6% (vs 48.8% vehicle) of participants in the VUITY® group had 20/40 to 20/60 mesopic, high-contrast binocular DCNVA.
In a post-hoc analysis:5
1/3 VUITY® participants achieved
20/20 DCNVA
without losing more than 5 letters
of CDVA on Day 30, Hour 1^
- 33.5% in GEMINI 1 vs 7.8% vehicle5
- 33.2% in GEMINI 2 vs 13.6% vehicle5
^This was not a prespecified endpoint; results could represent chance findings and should be interpreted with caution.
Statistically significant
improvement in DCIVA
vs vehicle
Prespecified secondary endpointChange from baseline in photopic, high-contrast binocular DCIVA letters at Day 30, Hour 3 (ITT population).3 At Day 30, Hour 3 (prespecified secondary endpoint)3 Mean change from baseline photopic DCIVA letters: GEMINI 1 was 6.6 letters with VUITY® vs 3.1 vehicle, p<0.05 (mean baseline of 53.4 letters in both groups). GEMINI 2 was 6.4 letters with VUITY® vs 3.1 vehicle, p<0.05 (mean baseline of 54.1 letters vs 54.4 letters, respectively).
NEARLY 9/10
Trial participants [N=76] surveyed would ask their eye doctor for VUITY®§7
§Assessed by a survey in a subset of participants in the VUITY® arm of GEMINI 1 and GEMINI 2 studies.
Not an actual patient
VUITY® has been studied in
two Phase 3 clinical trials1
Multicenter, double-masked, randomized, parallel-group, vehicle-controlled, 30-day Phase 3 studies in patients with presbyopia aged 40 to 55 years of age with mesopic, high-contrast DCNVA of 20/40 (J3) to 20/100 (J10) in each eye and at screening and baseline visits.
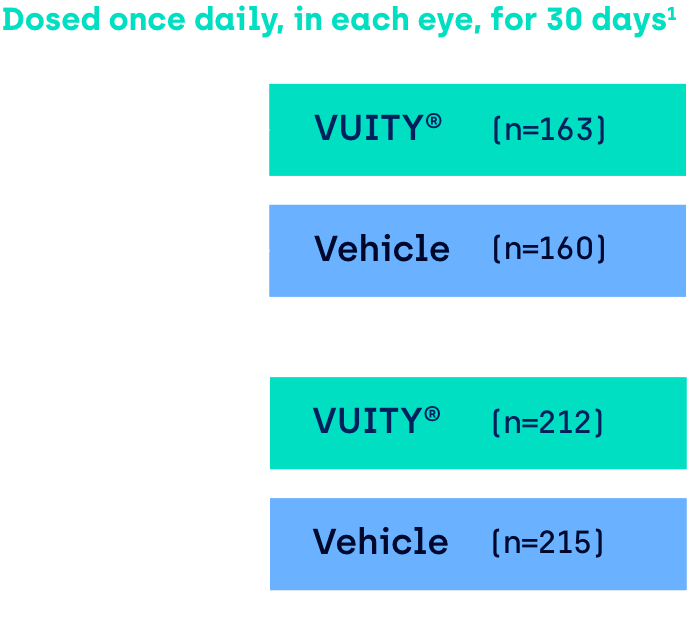
Study assessments:
Participants were evaluated on Day 1, 3, 7, 14 and 30 of the 30-day clinical trial.8
Primary efficacy assessment:
Percentage of participants in the ITT population gaining 3 lines or more from baseline in mesopic, high-contrast, binocular DCNVA without losing more than 1 line (5 letters) of CDVA with the same refractive correction at Day 30, Hour 3.1


